pf3 electron pair geometry|electron pair geometry chart : Clark The geometrical structure of the tetra-atomic Phosphorus Trifluoride (PF3) molecule is studied with the help of the Valence Shell Electron Pair Repulsion (VSEPR) theory. This theory explains that the bond angle between the fluorine-phosphorus-fluorine (F-P-F) is 97°. This angle makes . Tingnan ang higit pa From the minute I landed in Turkey the service I received was excellentCara, UK, 07 12 23. Absolutely love my new smile. When I started my enquiries back in the UK this clinic stood out from the rest, they weren’t pushy for a commitment, they were as thorough as possible with the quote from pictures, always available with my queries and most of all calmed my .
pf3 electron pair geometry,The geometrical structure of the tetra-atomic Phosphorus Trifluoride (PF3) molecule is studied with the help of the Valence Shell Electron Pair Repulsion (VSEPR) theory. This theory explains that the bond angle between the fluorine-phosphorus-fluorine (F-P-F) is 97°. This angle makes . Tingnan ang higit paAs per this rule, the maximum number of valence electrons an atom can have is eight. One phosphorus atom has five valence . Tingnan ang higit pa
The electrons present in the outermost shell of an atom are called valence electrons. Because they are present in the outermost shell, the hold of the nucleus is weak on them. Moreover, uneven or unpaired electrons compel them to participate . Tingnan ang higit paHybridization is a method of combining atomic orbitals of the same atom to produce new orbitals which are called hybrid orbitals. To figure out the hybridization of the central atom, it is essential to determine the steric number in the . Tingnan ang higit paThe Lewis structure is drawn using eight dots of valence electrons around the symbols of the atom with lines showing bond formation. PF3 is a tetra-atomic molecule . Tingnan ang higit pa
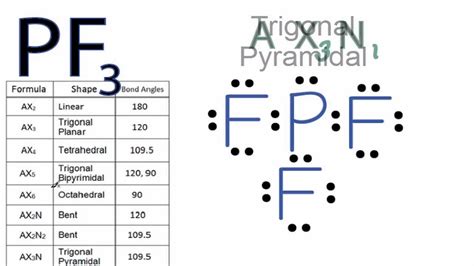
For the molecular geometry, looking at the PF3 Lewis structure we can see that there are three Fluorine (F) atoms attached to the central Phosphorus (P) atom and . In PF3 molecule electron density lies around the central Phosphorus atom and there are three bond pairs and one lone pair are present. PF3 lewis structure. In . A quick explanation of the molecular geometry of PF3 including a description of the PF3 bond angles. Looking at the PF3 Lewis structure we can see that . The molecular geometry or shape for PF3 is the trigonal pyramid. The electron geometry for PF3 is tetrahedral as it central has 4 regions of electron density. . Determine Electron Pair Geometry. Using VSEPR (Valence Shell Electron Pair Repulsion) theory, the electron pairs around the central atom (phosphorus) will . 6 Steps to Draw the Lewis Structure of PF3 Step #1: Calculate the total number of valence electrons. Here, the given molecule is PF3 (phosphorus trifluoride). In . We show you how to draw the Lewis structure and determine the moleculargeometry for phosphorus trifluoride (PF3).
pf3 electron pair geometry electron pair geometry chartIn calculating electronic geometry we use the Valence Shell Electron Pair Repulsion (VSEPR) model, which states that the lowest geometry for electronic orbitals around a positive nucleus is for the orbitals to be as .
pf3 electron pair geometry The three F atoms bond to the P, but this leaves a lone pair as the fourth electron pair in the valence shell of phosphorus. These nonbonding electrons repel the .The Lewis structure for PF3 is shown. What is the electron-pair geometry around the central atom? :F: Electron Pair Geometry: Table 1.1 Basic VSEPR Shapes. Notes: . For VSEPR purpose, the terms “shape” and “geometry” are interchangeable; “electron pair” and “electron group” are also interchangeable. Multiple bonds . Yes, PF3 is tetrahedral in shape. According to the VSEPR theory, the presence of three bonding pairs and one lone pair of electrons on the Phosphorus atom results in a tetrahedral electron pair geometry. 6. Does PF3 dissolve in water? PF3 does not dissolve well in water.Our expert help has broken down your problem into an easy-to-learn solution you can count on. Question: Draw the Lewis structure for PF3 (phosphorous is the central atom) and select the correct model representing its "electron pair geometry' and 'molecular geometry". Electron Pair Geometries Unear Trigonal planar Tetrahedral Tetrahedral . The electron geometry of PF3 is determined by the steric number, which is the sum of bonded atoms and lone pairs of electrons around the central atom. Phosphorus has five valence electrons and makes three bonds with fluorine atoms, leaving behind one lone pair on the phosphorus. Thus, PF3 has four areas of electron density.
Phosphorus Trichloride: Phosphorus trifluoride is the name of PF 3.It's a gas that is known for its toxicity. We will use valence shell electron pair repulsion (VSEPR) theory to determine its molecular geometry.
Then, with the Lewis structure, we apply the valence-shell electron-pair repulsion (VSPER) theory to determine the molecular geometry and the electron-group geometry. To identify and have a complete description of the three-dimensional shape of a molecule, we need to know also learn about state the bond angle as well. The VSEPR shape of the molecule PF3 is trigonal pyrimidal. We can use VESPR theory to predict a trigonal pyrimidal shape for the molecule P F 3 because of its AX3E status. VESPR stands for valence shell electron pair repulsion. This theory basically says that bonding and non-bonding electron pairs of the central atom in a molecule will .Trigonal Bipyramidal Electron Geometry. A central atom with five pairs of bonding electron pairs is known as trigonal bipyramidal. It has the shape of three pairs in a plane at 120° angles (the trigonal planar geometry) and the remaining two pairs at 90° angles to the plane. The shape is similar to two pyramids joined by a triangular base. The total valence electron available for the NF3 lewis structure is 26. Hybridization of NF3 is Sp³. NF3 is polar in nature. The molecular geometry or shape of NF3 is a trigonal pyramid and its electron geometry is tetrahedral. NF3 lewis dot structure contains 1 lone pair and 3 bonded pairs. Very important. Clarify which type of geometry the question is asking about before answering it. The electron domain geometry is tetrahedral for both but the molecular geometry is trigonal pyramidal (not trigonal bipyramidal for PF3) for both. Both geometrical assignments are due to the lone pair of electrons on the central atom(s) of .

Example \(\PageIndex{1}\) Determine the Electron Group Arrangement and Molecular Geometry about the central atom(s) in a) OF 2 and b) CH 3 CN.. Solution. a) The Lewis dot structure of OF 2 is (leaving off the lone pairs on the non-central F atoms.). There are 2 atoms and 2 lone pairs attached to the central O atom, for a total of 4 "things .

So we have to only mark the remaining ten electron pairs as lone pairs on the sketch. Also remember that phosphorus is a period 3 element, so it can keep more than 8 electrons in its last shell. And fluorine is a period 2 element, so it can not keep more than 8 electrons in its last shell. Always start to mark the lone pairs from outside atoms.electron pair geometry chart Also, only 24 valence electrons of PF3 molecule are used in the above structure. But there are total 26 valence electrons in PF3 molecule (as calculated in step #1). So the number of electrons left to be kept on the central atom = 26 – 24 = 2. So let’s keep these two electrons (i.e electron pair) on the central atom.
Question: PF3 Bonding Atoms: Non-Bonding Electron pairs (central atom): Electron Pair Geometry: Select an answer Molecular Shape: Select an answer Polar: yes :F: :F—P P—F: Show transcribed image text. There are 3 steps to . The geometry of BCl3 BCl 3 is also given in Figure 7.2: it is trigonal planar, with all four atoms lying in the same plane, and all Cl−B−Cl Cl − B − Cl bond angles equal to 120o 120 o. The three Cl Cl atoms form an equilateral triangle. The Boron atom has only three pairs of valence shell electrons in BCl3 BCl 3.
The electron pair geometry and the molecular structure of each are as follows: Number of valence electrons: [latex]\ce{S}[/latex] = 6, [latex]\ce{F}[/latex] = 7 each, total 48. A single line bond represents two electrons: The total number of electrons used is 48; six bonds are formed and no nonbonded pairs exist. Therefore the molecule includes .
5.4: VSEPR Geometry is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. The Lewis electron-pair approach described previously can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. ..
pf3 electron pair geometry|electron pair geometry chart
PH0 · pf3 lewis structure molecular geometry
PH1 · how to determine electron geometry
PH2 · electron pair geometry chart
PH3 · electron geometry vs molecular geometry
PH4 · Iba pa